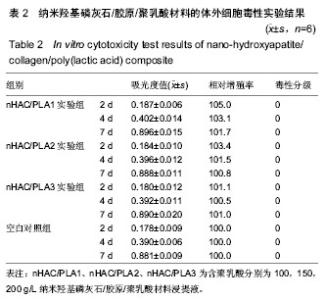
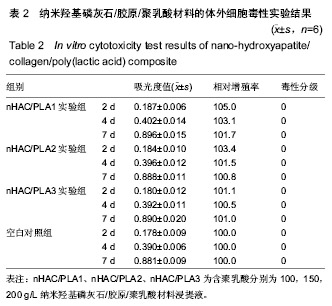
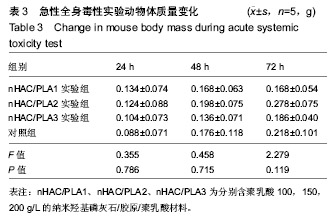
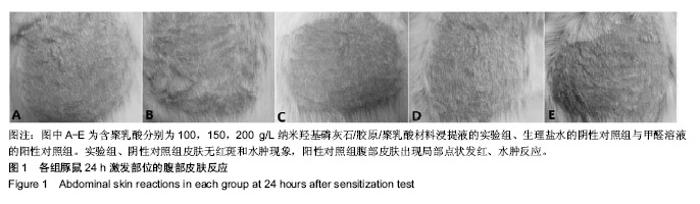
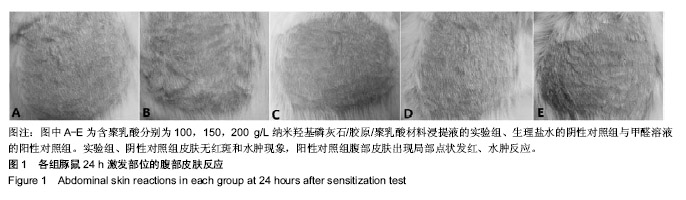
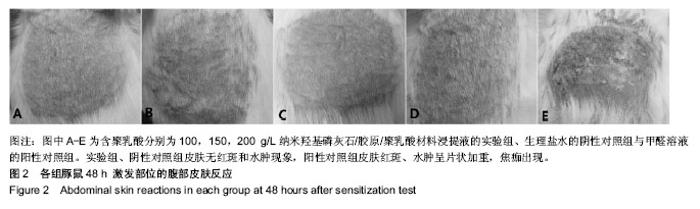
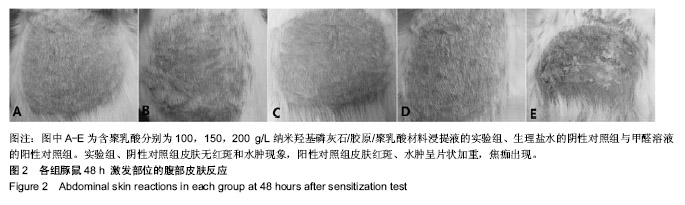
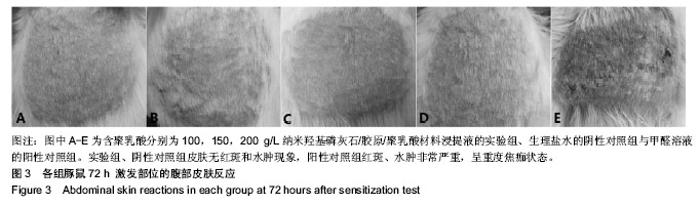
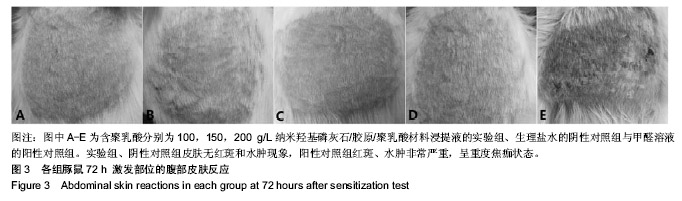
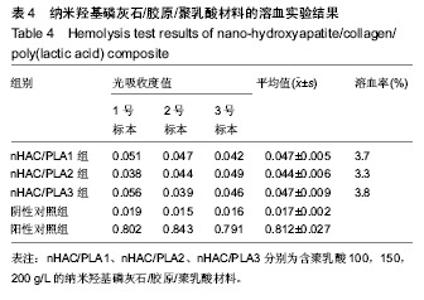
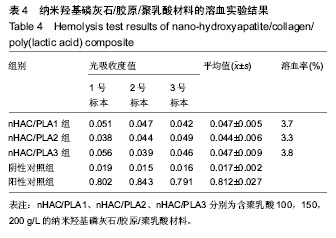
| [1]Makarawo TP,Reynolds RA,Cullen ML.Polylactide bioabsorbable struts for chest wall reconstruction in a pediatric patient.Ann Thorac Surg.2015;99(2):689-691.[2]Félix Lanao RP,Jonker AM,Wolke JG,et al.Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration.Tissue Eng Part B Rev. 2013;19(4): 380-390.[3]Hanna LA,Basalious EB,Elgazayerly ON.Respirable controlled release polymeric colloid (RCRPC) of bosentan for the management of pulmonary hypertension: in vitro aerosolization, histological examination and in vivo pulmonary absorption.Drug Deliv.2017; 24(1):188-198.[4]章越,丁陈陈,温露,等.核壳型聚乳酸-羟基乙酸共聚物磁性纳米系统用于中药复方多组分的时空递释[J].药学学报, 2018,53(12): 1968-1975.[5]Libonati F,Nair AK,Vergani L,et al.Fracture mechanics of hydroxyapatite single crystalsunder geometric confinement.J Mech Behav Biomed Mater.2013;20:184-191.[6]Venkatesan J,Kim SK.Nano-Hydroxyapatite Composite Biomaterials for Bone TissueEngineering-A Review. J Biomed Nanotechnol.2014;10(10):3124-3140.[7]Liao SS,Cui FZ,Zhang W,et al.Hierarchically biomimetic bone scaffold materials: Nano- HA/ collagen/PLA composite.J Biomed Mater Res B Appl Biomater.2004;69(2):158-165.[8]Bhuiyan DB,Middleton JC,Tannenbaum R,et al.Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds. Biomed Mater Eng.2017;28(6):671-685.[9]ISO 10993-5:2014 Biological evaluation of medical devices, Part 5: Tests for in vitro cytotoxicity.[10]ISO 10993-1:2010 Biological evaluation of medical device, Part 1: Guidance on selection of tests.[11]SO 10993-12:2012 Biological evaluation of medical device, Part 12: Sample preparation and reference materials.[12]杨杨,季娟娟,彭蓓,等.MTT法评价4种铸造合金的细胞毒性研究[J].临床口腔医学杂志,2008,24(11):649-651.[13]Nakamura M,Kawahara H,Kataoka Y,et al.Biocompatibility of dental amalgams in vitro during 52 week period.Shika Rikōgaku Zasshi.1980;21(55):228-244.[14]ISO 10993-11:2010 Biological evaluation of medical device, Part 11: Tests for systemic toxicity.[15]沈晴昳,李国强,李恺.新型复合磷酸钙镁基水门汀的生物相容性[J].中国组织工程研究,2018,22(2): 241-247.[16]张桥.卫生毒理性研究[M].北京:人民卫生出版社,2001:236-246.[17]ISO/TR 7405-1984(E).Haemolysis test(in vitro test directly on materials).[18]Yeon YK,Park HS,Lee JM,et al.New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J Biomater Sci Polym Ed. 2018;29(7-9):894-906.[19]Wang X,Zhang GL,Qi F,et al.Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/PLGA composite scaffold.Int J Nanomedicine.2017;13:117-127.[20]史建国,王卫宁,李春吾,等.脂肪干细胞复合生物可降解材料体外构建组织工程尿道支架[J].中国组织工程研究, 2019,23(13): 1989-1994.[21]Chienb BW,Ibrahim NA,Wan MZWY,et al.Plasticized poly(lactic acid)with low molecular weight poly(ethylene glycol):mechanical,thermal,and morphology properties.J Appl Polym Sci.2013;130(6):4576-4580.[22]Rasal RM,Janorkar AV.Poly(lactic acid) modifications.Prog Polym Sci.2010;35(3):338-356.[23]Lee BK,Yun Y,Park K.PLA micro- and nano-particles. Adv Drug Deliv Rev.2016;107(15):176-191.[24]Du C,Cui FZ,Zhang W,et al.Formation of calcium phosphate/collagen composites through mineralization of collagen matrix.J Biomed Mater Res.2000;50(4):518-527.[25]Hao HP. Biological Evaluation of Medical Devices Implementation Guide. Beijing : Standards Press of China, 2000.[26]De SC,Hebling J,Scheffel DL,et al.Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques.Dent Mater.2014;30(7):769-784.[27]Murni NS,Dambatta MS,Yeap SK,et al. Cytotoxicity evaluation of biodegradable Zn-3Mg alloy toward normal human osteoblast cells.Mater Sci Eng C Mater Biol Appl. 2015;49: 560-566.[28]Cui X,Liang T,Liu C,et al.Correlation of particle properties with cytotoxicity and cellular uptake of hydroxyapatite nanoparticles in human gastric cancer cells.Mater Sci Eng C Mater Biol Appl. 2016;67:453-460.[29]Ong THD,Yu N,Meenashisundaram GK,et al.Insight into cytotoxicity of Mg nanocomposites using MTT assay technique. Mater Sci Eng C Mater Biol Appl.2017;78:647-652.[30]Heaney ML,Golde DW.Soluble cytokine receptors.Blood. 1996;87(3):847-857. |